An introduction for students, explaining the principles and offering demonstrations
What is electroplating?Electroplating (often just called "plating") is the deposition of a metal coating onto an object by putting a negative charge on it and putting it into a solution which contains a metal salt. The metal salt contains positively charged metal ions which are attracted to the negatively charged object and are "reduced" to metallic form upon it. How does plating work?
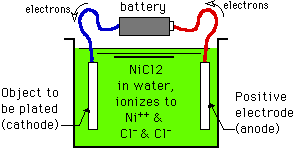
Look at the figure above: We have a metal object that we want to plate with another metal. First we fill a "cell" (a tank, vat, or bowl) with a solution of a salt of the metal to be plated. Most of the time the salt (nickel chloride in our example) is simply dissolved in water and maybe a little acid. In this example, the NiCl2 salt ionizes in the water into Ni++ ions and two parts of Cl- ions. A wire is attached to the object, and the other end of the wire is attached to the negative pole of a battery (with the blue wire in this picture) and the object is immersed in the cell. A rod made of nickel is connected to the positive pole of the battery with the red wire and immersed in the cell. The battery is pulling electrons away from the nickel anode (through the red wire) and pumping them over to the object to be plated (through the blue wire) Because the object to be plated is negatively charged (by being connected to the negative pole of the battery and having electrons pumped to it), it attracts the positively charged Ni++ ions that are floating around in the solution. These Ni++ ions reach the object, and electrons flow from the object to the Ni++ ions. For each ion of Ni++, 2 electrons are required to neutralize its positive charge and "reduce" it to an atom of Ni0 metal. Thus, the amount of metal that deposits is directly proportional to the number of electrons that the battery provides. Note: This proportional relationship is a reflection of Faraday's Law of Electrolysis. If you are advanced enough in chemistry (a high school student), that you've heard terms like gram molecular weight, mole, valence and Avagadro's number, but it's all a hodgepodge to you instead of a cohesive whole, don't despair! Study Faraday's Law for a while, which says that 96,500 coulombs of electricity will deposit one gram equivalent weight of metal, and all of these disparate wacky terms will come together in a moment of enlightenment. |
||||